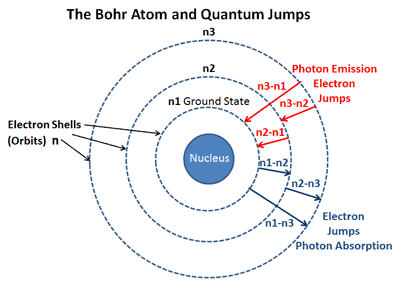
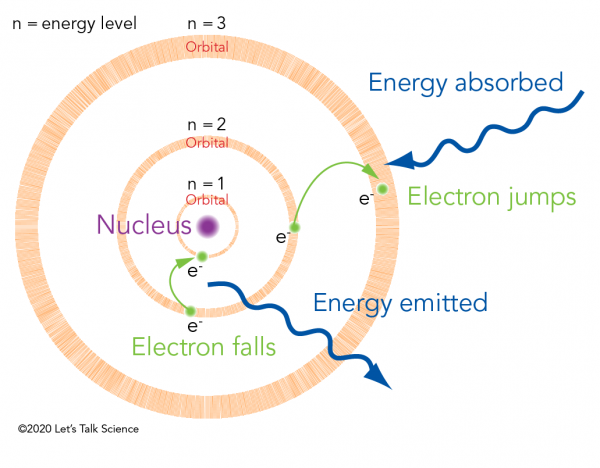
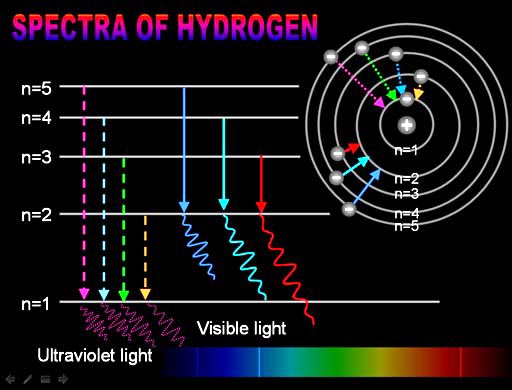
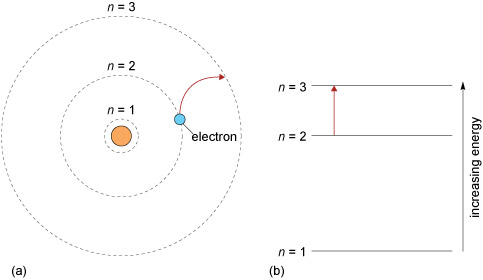
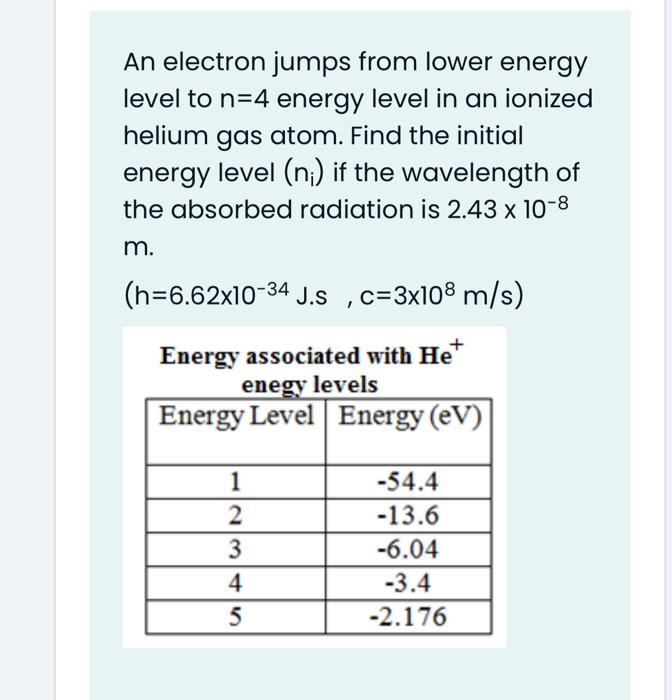
To which quantum level does the electron jump in H atom from the lowest level it is given an energy corresponding to 99% of the ionisation potential?
When an electron jumps from a higher orbit to a lower orbit, how does it know what amount of energy it must lose to land into another allowed state? - Quora
illustration of chemistry and physics, Quantum leap, Photon of light hits electron, Electron jumps to higher energy shell, quantum transition Stock-Vektorgrafik | Adobe Stock
If an electron jumps from the n=2 orbit to the n=3 orbit, then what kind of spectrum will be produced: a continuous spectrum, an absorption line spectrum, or an emission line spectrum?

Quantum Science for standard 10 to 12, Innovative technique and Animation Free download picture: Electron jump.
Can the hydrogen electron jump from level n=3 to level n=1 during emission of visible light? - Quora